
Test system for a new generation of sensors
Development of a test system for leak and flow testing of new sensors.

Development of a multi-unit system for dental implants
Development of a market-proven multi-unit system for dental implants.

Test bench for high-pressure direct room humidification system
Test bench for the Condair Vita platform: automated testing for highest quality and efficiency.

Product adaptations and UL certification for a spa control system
Targeted product adaptations and UL certification allowed successful entry into the US market for Condair.

Extended PLCopen Safety Modules – standard-compliant and future-proof
Optimizing a PLCopen Safety Library: standard-compliant extended, verified, and validated - future-proof for industrial automation.

EMC design for IoT products
Optimization of EMC design for IoT-capable products – from analysis to final EMC testing.

Quality management system for pharmaceuticals and medical devices
New quality management system for pharmaceuticals and medical devices strengthens the healthcare division of our customer.
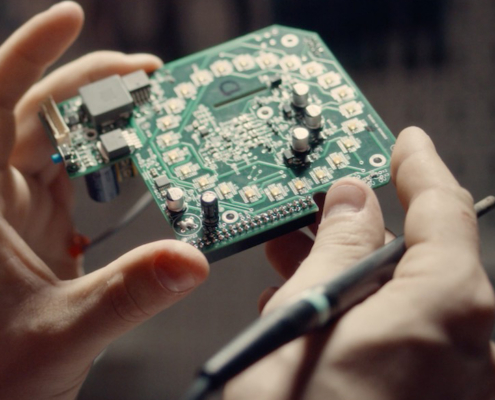
KITRO’s Vision for Industrialization
Industrial-grade hardware and smart software for KITRO's food waste system.
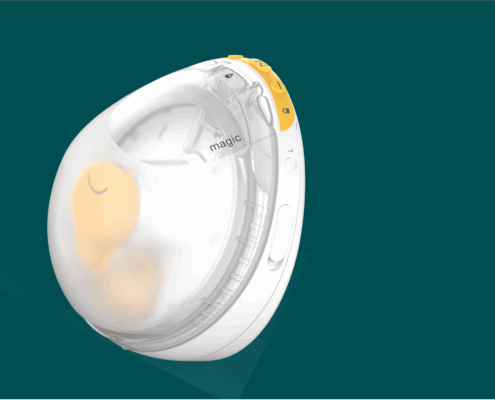
Mobile app for controlling a new type of breast pump
Conception and implementation of a mobile app for Medela's first InBra breast pump.

Tool interface for haptic simulator
Expansion of the haptic interface and integration of sensor technology into various tools for simulating endoscopic procedures.

Secure firmware over-the-air update for sterilizers and cleaning devices
A feasibility study provided Belimed with a comprehensive view of the architecture and implementation of the secure firmware over-the-air update feature for the current system.

With efficient sprints to country approvals
Country approvals efficiently achieved with standardized sprint processes and the bundling of changes.

