Posts

With efficient sprints to country approvals
Country approvals efficiently achieved with standardized sprint processes and the bundling of changes.

Change management for an eye surgery device in development
Ensuring that changes to the eye surgery device in series production comply with regulatory requirements.
Change coordination and administrative support
The introduction of a standard change process and the creation of technical documentation ensured efficient project progress.
Document Creation for UL Certification
Support in the preparation of a List of Critical Components for UL certification up to system data comparison.
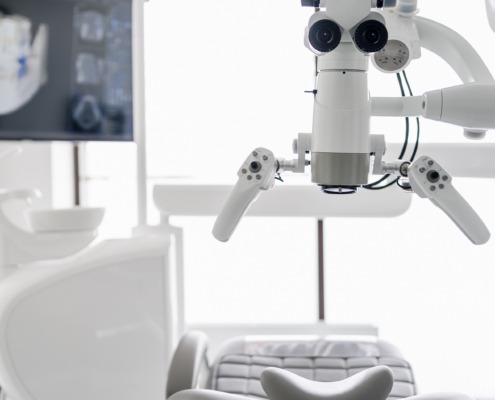
Self-stabilizing microscope articulating arm
Functional model of a self-stabilizing microscope articulating arm system for medical diagnosis and the treatment of various diseases.
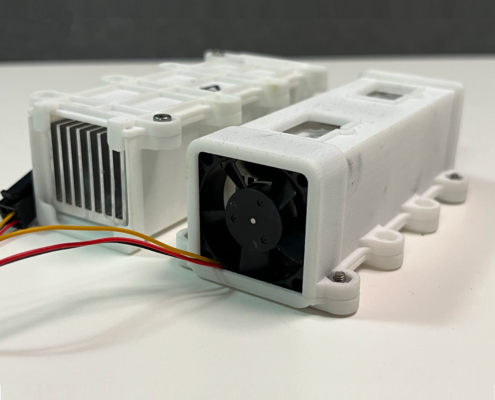
Optimizing the temperature control for a microfluidic cartridge
As part of the new design of a multiparameter analysis device, the temperature control for the microfluidic cartridge was developed.

Development of a microfluidic cartridge for an analytical device
After thorough material evaluation, the feasibility studies for a microfluidic cartridge of an analytical device were successfully verified.
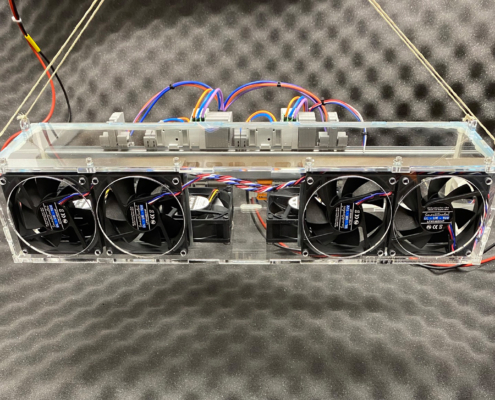
Development of the device cooling for a robotic visualization system
Through calculation, simulation, construction, and prototype construction, an optimal system design for the device cooling was developed.

Internationalization of User Interfaces
How developing a translation plugin to internationalize user interfaces leads to shorter development times.

Bluetooth interface test system for mobile diagnostic devices
How the development of a Bluetooth interface test system can ensure early problem detection even before market launch.
 https://konplan.com/wp-content/uploads/2018/07/konplan_Juli_in_4-1-e1626439718965.jpg 627 1200 Tonazzi dot net https://konplan.com/wp-content/uploads/konplan_logo_quer_gruen_2024.svg Tonazzi dot net2018-07-26 08:50:142018-07-26 08:50:14Gesetze und Normen 2018 – Auswirkungen für die Cyber-Sicherheit von Medizintechnik
https://konplan.com/wp-content/uploads/2018/07/konplan_Juli_in_4-1-e1626439718965.jpg 627 1200 Tonazzi dot net https://konplan.com/wp-content/uploads/konplan_logo_quer_gruen_2024.svg Tonazzi dot net2018-07-26 08:50:142018-07-26 08:50:14Gesetze und Normen 2018 – Auswirkungen für die Cyber-Sicherheit von Medizintechnik  https://konplan.com/wp-content/uploads/2018/04/konplan_blog_FDA_IN_2-e1626439766435.jpg 627 1200 Tonazzi dot net https://konplan.com/wp-content/uploads/konplan_logo_quer_gruen_2024.svg Tonazzi dot net2018-04-19 17:00:462021-07-22 12:55:47FDA 21 CFR Part 11 – elektronische Aufzeichnungen und elektronische Unterschriften
https://konplan.com/wp-content/uploads/2018/04/konplan_blog_FDA_IN_2-e1626439766435.jpg 627 1200 Tonazzi dot net https://konplan.com/wp-content/uploads/konplan_logo_quer_gruen_2024.svg Tonazzi dot net2018-04-19 17:00:462021-07-22 12:55:47FDA 21 CFR Part 11 – elektronische Aufzeichnungen und elektronische Unterschriften
