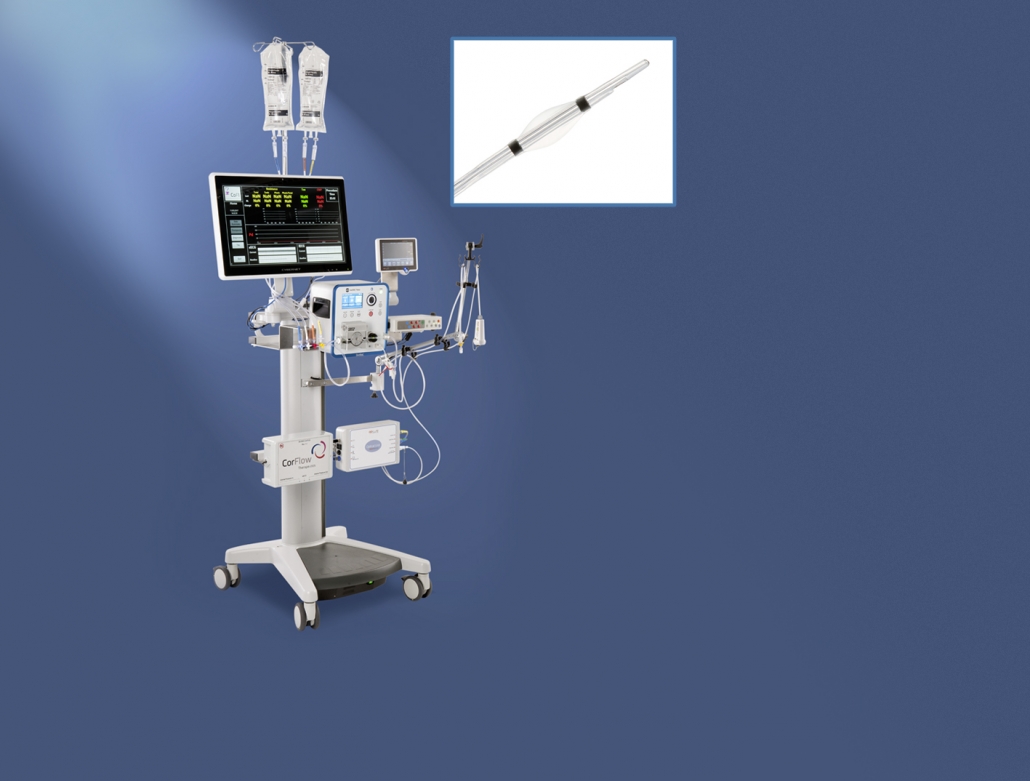
Console for Controlled Flow Infusion (CoFITM)
CorFlow Therapeutics AG is a medtech startup based in Zug that is developing a new type of therapy for diagnosis and treatment of “microvascular obstructions” after a heart attack. Using this new type of therapy allows cardiologists to reduce the short-term and long-term complications that occur after a heart attack.
In the initial phase, CorFlow needed a cost-effective hardware solution to validate its new type of therapy in preclinical studies. The next phase was focused on verification and generating the proper documentation for clinical validation. The resulting prototypes needed to be designed to allow therapeutic intervention to be performed in parallel with real-time monitoring and diagnosis of the patient’s microvascular condition.
konplan supported CorFlow from the conception phase and worked closely with the customer to define the system requirements and specifications of the device. With this information, konplan created the applicable system architecture, evaluated suppliers for the required components, and developed the software for the user interface. The devices were assembled and verified in-house by konplan.
For rapid software development, the data streams of the various components were combined using NI technology (LabVIEW). This allowed the measured values and the treatment process to be clearly presented.
As a comprehensive engineering service company, konplan was able to provide CorFlow with everything from a single source including conception, selection of components and supplier, software development, and prototype assembly.
konplan also generated the proper documentation and verification processes to make sure that the development of the devices was compliant with regulatory requirements. This allowed immediate use of the devices in a multicenter clinical study.