Development of a thermotherapy device for the treatment of cutaneous leishmaniasis
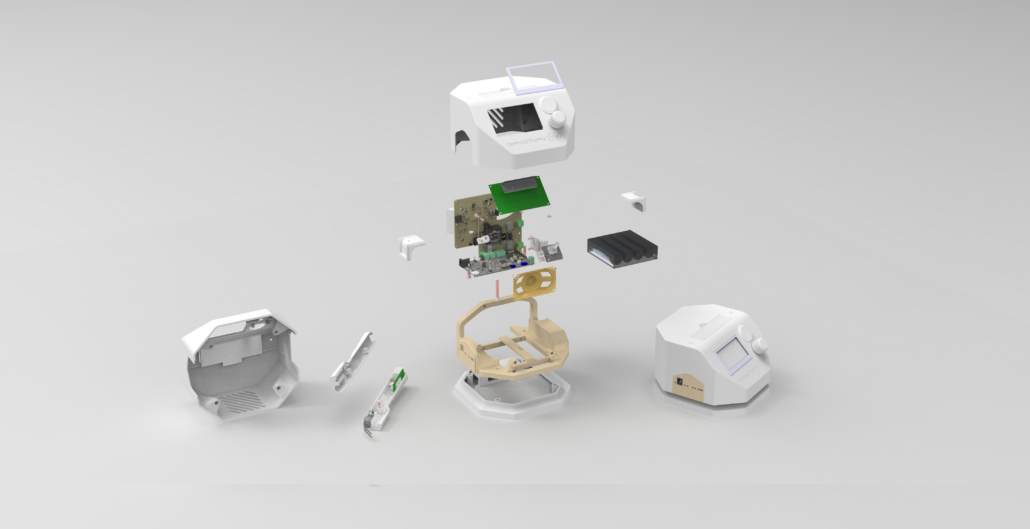
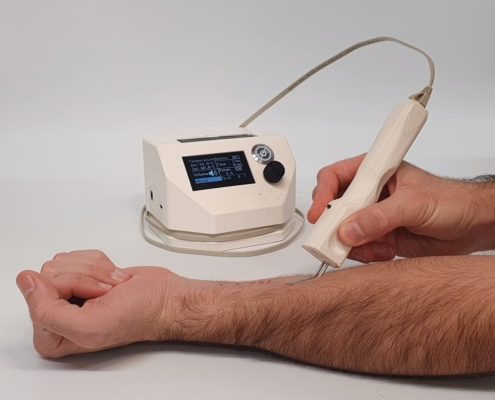
The founders of DermatoTherma have developed a cost-effective thermotherapy device in the form of a demonstrator that can be used to treat cutaneous leishmaniasis disease. Their mission is to provide the general population with a method of treatment for the disease in countries where it is endemic. In collaboration with konplan, the existing demonstrator is to be developed into a medical device and prepared for FDA approval.
Development according to MedTech standards
In the beginning, konplan supported setting up the quality management system for DermatoTherma according to the ISO 13485 medical device standard which lays the foundation for MedTech development. Next, in the first phase of development, the team used close collaboration and workshops to define the design input. The specialists prepared the basic development documents according to the MedTech standard including the stakeholder requirements, design requirements, risk analysis, development plan, and risk management plan. Based on the results, konplan developed a system architecture with critical design decisions and risk mitigations. The documentation was created according to ISO 13485 with proper review and approval processes and the support of Qualio tools.
A safe product thanks to a risk-based approach
From the beginning, a risk-based approach was considered paramount. It is an important fundamental of MedTech product development where patient safety must be guaranteed. This was always considered in the development of concepts and system architecture. Completion of the first phase provided DermatoTherma a good basis for further developing the product according to MedTech standards.